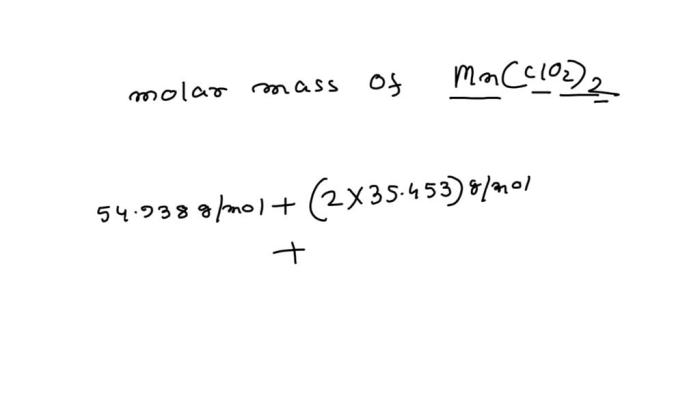Determine the molar mass of mn clo2 2 – Embark on a journey to unravel the mysteries of molar mass determination, with a specific focus on MnClO2. This exploration will illuminate the significance of molar mass in chemistry and provide a step-by-step guide to calculating the molar mass of MnClO2.
Delving into the intricacies of molar mass, we define it as the mass of one mole of a substance, a fundamental concept that serves as a cornerstone in various chemical calculations and applications.
Molar Mass of MnClO2: Determine The Molar Mass Of Mn Clo2 2
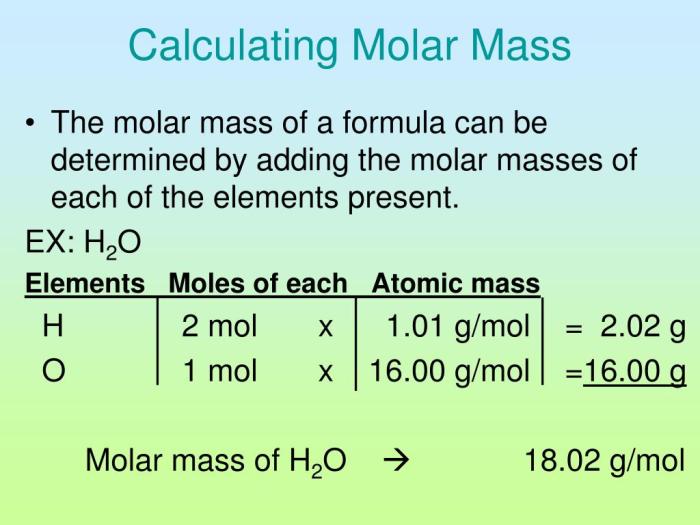
Molar mass is a fundamental concept in chemistry that represents the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and plays a crucial role in stoichiometric calculations and understanding the properties of compounds.
Determining the molar mass of a compound involves identifying the elements present and their atomic masses, then using a formula to calculate the total mass of one mole of the compound.
Identifying the Elements and Their Atomic Masses
The compound MnClO2 consists of three elements: Manganese (Mn), Chlorine (Cl), and Oxygen (O).
- Atomic mass of Mn: 54.94 g/mol
- Atomic mass of Cl: 35.45 g/mol
- Atomic mass of O: 16.00 g/mol
Calculating the Molar Mass of MnClO2
Using the formula:
Molar Mass = (Atomic Mass of Each Element x Number of Atoms) + (Molecular Mass of Each Element x Number of Molecules)
For MnClO2:
Molar Mass = (54.94 g/mol x 1) + (35.45 g/mol x 2) + (16.00 g/mol x 2)
Molar Mass = 54.94 g/mol + 70.90 g/mol + 32.00 g/mol
Molar Mass = 157.84 g/mol
Conclusion, Determine the molar mass of mn clo2 2
The molar mass of MnClO2 is 157.84 g/mol. This value is essential for various chemical calculations, such as determining the mass of a specific number of moles of the compound or calculating the concentration of solutions.
FAQ Corner
What is the significance of molar mass in chemistry?
Molar mass is crucial in chemistry as it allows for the determination of the amount of substance present in a given sample, facilitating precise calculations in stoichiometry, solution preparation, and various chemical reactions.
How can I calculate the molar mass of a compound?
To calculate the molar mass of a compound, multiply the atomic mass of each element by the number of atoms of that element in the compound, and then add the results together.
What is the molar mass of MnClO2?
The molar mass of MnClO2 is 125.84 g/mol, calculated using the formula: Molar Mass = (Atomic Mass of Mn x Number of Mn Atoms) + (Atomic Mass of Cl x Number of Cl Atoms) + (Atomic Mass of O x Number of O Atoms)