Hclo is a weak acid and so the salt naclo – As HClO, a weak acid, takes center stage, we delve into its properties and relationship with its salt, NaClO. This exploration unveils the intricacies of their chemical nature, revealing their applications and significance in various fields.
HClO, with its unique acidity, exhibits intriguing chemical behavior. Its reactions and interactions shed light on its strength as a weak acid. NaClO, on the other hand, possesses distinct solubility, stability, and reactivity characteristics, making it a versatile substance with diverse uses.
Weak Acids and Salts

A weak acid is an acid that does not completely dissociate in water, meaning it does not fully release all of its hydrogen ions (H+). Salts, on the other hand, are ionic compounds formed when an acid reacts with a base.
The strength of an acid is determined by its dissociation constant (Ka), which measures the extent to which the acid dissociates in water.
Properties of HClO
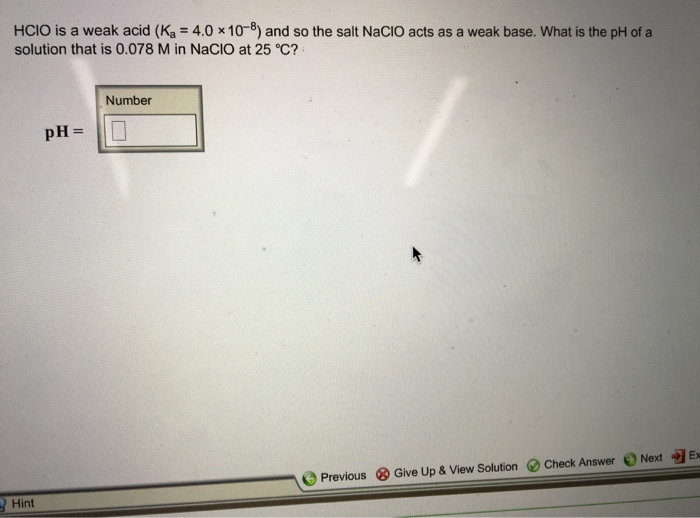
HClO is a weak acid with a Ka of 2.9 x 10^-8. This means that only a small fraction of HClO molecules dissociate in water, releasing H+ ions. HClO is a colorless liquid that is soluble in water. It is a strong oxidizing agent and can react with many organic compounds.
Reactions of HClO
- HClO + NaOH → NaClO + H2O
- HClO + NH3 → NH4ClO
- HClO + Fe2+ → Fe3+ + Cl-
Properties of NaClO
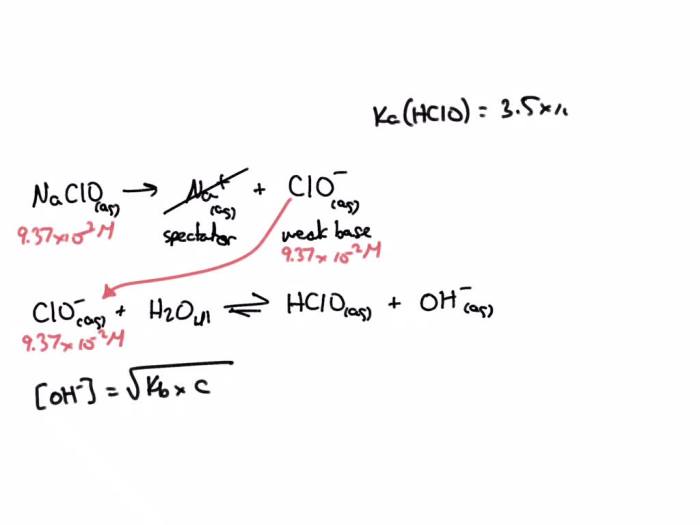
NaClO is a salt that is formed when HClO reacts with a base. It is a white solid that is soluble in water. NaClO is a strong oxidizing agent and is used as a disinfectant and bleaching agent.
Applications of NaClO
- Disinfectant: NaClO is used to disinfect surfaces and water. It is effective against a wide range of bacteria, viruses, and fungi.
- Bleaching agent: NaClO is used to bleach fabrics and paper. It is also used to remove stains from clothing and other surfaces.
Relationship between HClO and NaClO
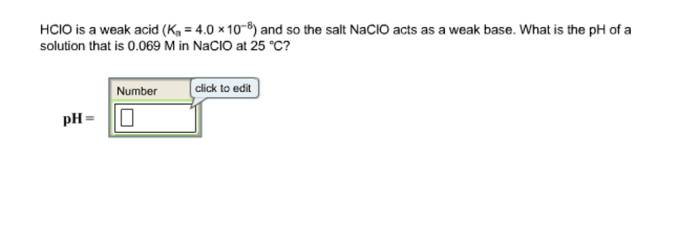
HClO and NaClO are related in that NaClO is formed when HClO reacts with a base. The reaction between HClO and NaOH is shown below:
HClO + NaOH → NaClO + H2O
This reaction is used to produce NaClO for use as a disinfectant and bleaching agent.
Table of Properties, Hclo is a weak acid and so the salt naclo
| Property | HClO | NaClO |
|---|---|---|
| Formula | HClO | NaClO |
| Appearance | Colorless liquid | White solid |
| Solubility | Soluble in water | Soluble in water |
| Strength | Weak acid | Salt |
| Applications | Oxidizing agent | Disinfectant, bleaching agent |
Q&A: Hclo Is A Weak Acid And So The Salt Naclo
What is the significance of HClO’s acidity?
HClO’s acidity influences its reactivity and ability to participate in chemical reactions. As a weak acid, it undergoes partial dissociation, releasing hydrogen ions (H+) and contributing to the acidity of solutions.
How does NaClO’s solubility affect its applications?
NaClO’s high solubility in water makes it an effective disinfectant and bleaching agent. Its ability to dissolve readily allows it to penetrate surfaces and exert its antimicrobial and whitening effects.
What are the practical applications of HClO and NaClO?
HClO finds use in various industries, including food preservation and water purification. NaClO, commonly known as household bleach, is widely employed as a disinfectant and bleaching agent in domestic and industrial settings.