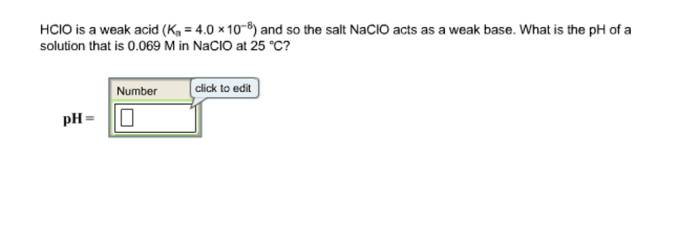
Step into the realm of chemistry with the Chemistry Moles Packet Answer Key, your trusted companion in unraveling the mysteries of moles. As the foundation of quantitative chemistry, moles play a pivotal role in understanding the composition and behavior of substances.
This comprehensive guide delves into the intricacies of moles, equipping you with the knowledge to conquer chemistry’s challenges.
From calculating the number of moles in a substance to using moles in stoichiometric calculations, this guide covers it all. Explore the relationship between moles and mass, molarity, and gas laws. Engage in practice problems to solidify your understanding and master the art of mole-based calculations.
Chemistry Moles Packet Answer Key Overview
The concept of moles is crucial in chemistry. A mole represents a specific amount of a substance, analogous to a dozen representing a specific number of objects. In chemistry, a mole is defined as the amount of a substance that contains exactly 6.022 × 10^23 elementary entities, which can be atoms, molecules, ions, or electrons.
Moles are used to express the quantity of a substance in a convenient and standardized manner. They allow chemists to determine the number of particles present in a sample, calculate the mass of a substance, and determine the volume of a gas under specific conditions.
Typical Questions Found in a Moles Packet
Moles packets typically include questions that test students’ understanding of the following concepts:
- Converting between mass and moles
- Calculating the molar mass of a compound
- Determining the number of moles of a substance in a given sample
- Calculating the concentration of a solution in moles per liter
- Balancing chemical equations using moles
Moles and Calculations: Chemistry Moles Packet Answer Key
In chemistry, a mole is a fundamental unit of measurement used to quantify the amount of a substance. It represents a specific number of entities, which can be atoms, molecules, ions, or electrons.
Calculating the Number of Moles
To calculate the number of moles in a substance, we divide its mass by its molar mass. Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). The formula for calculating the number of moles (n) is:
n = mass (g) / molar mass (g/mol)
Detailed Example
Let’s calculate the number of moles in 100 grams of sodium chloride (NaCl). The molar mass of NaCl is 58.44 g/mol.
n = 100 g / 58.44 g/moln = 1.71 moles
Relationship between Moles and Mass
The number of moles in a substance is directly proportional to its mass. This means that if we double the mass of a substance, we will also double the number of moles.
Stoichiometry and Moles
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It plays a crucial role in understanding and predicting the outcome of chemical reactions.
Moles are a fundamental unit used in stoichiometry to represent the amount of a substance. One mole of a substance is defined as the amount that contains exactly 6.022 x 10 23representative particles of that substance, which can be atoms, molecules, ions, or electrons.
Using Moles in Stoichiometric Calculations, Chemistry moles packet answer key
Moles are used extensively in stoichiometric calculations to determine the amount of reactants or products involved in a chemical reaction. The balanced chemical equation for a reaction provides the mole ratios between the reactants and products.
For example, consider the combustion of methane (CH 4) with oxygen (O 2) to produce carbon dioxide (CO 2) and water (H 2O):
CH 4+ 2O 2→ CO 2+ 2H 2O
The balanced equation indicates that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water. This information can be used to perform stoichiometric calculations involving these substances.
Solutions and Moles
Molarity is a measure of the concentration of a solution, specifically the number of moles of solute per liter of solution. It is expressed in units of moles per liter (mol/L). The relationship between molarity and moles is direct and proportional.
A solution with a higher molarity contains more moles of solute per liter compared to a solution with a lower molarity.Preparing solutions of a specific molarity involves dissolving a known mass of solute in a known volume of solvent. The mass of solute required can be calculated using the formula:“`Mass of solute (g) = Molarity (mol/L) × Volume of solution (L) × Molar mass of solute (g/mol)“`For example, to prepare 1 liter of a 1 M solution of sodium chloride (NaCl), you would dissolve 58.44 g of NaCl (the molar mass of NaCl is 58.44 g/mol) in 1 liter of water.
Titrations and Moles
Titration is a technique used in chemistry to determine the concentration of a solution by adding a known volume of a reagent to it until a reaction is complete. The point at which the reaction is complete is called the equivalence point.
Moles are used in titration calculations to determine the amount of substance that is present in a solution. The number of moles of a substance is equal to its mass divided by its molar mass.
Example of a Titration Problem Involving Moles
A 25.0 mL sample of a solution of HCl is titrated with a 0.100 M solution of NaOH. The equivalence point is reached after 20.0 mL of the NaOH solution has been added. What is the concentration of the HCl solution?
Step 1: Calculate the number of moles of NaOH used.
Moles of NaOH = Concentration of NaOH × Volume of NaOH
Moles of NaOH = 0.100 M × 0.0200 L
Moles of NaOH = 0.00200 mol
Step 2: Determine the number of moles of HCl that reacted with the NaOH.
Because the reaction between HCl and NaOH is 1:1, the number of moles of HCl that reacted with the NaOH is also 0.00200 mol.
Step 3: Calculate the concentration of the HCl solution.
Concentration of HCl = Moles of HCl / Volume of HCl
Concentration of HCl = 0.00200 mol / 0.0250 L
Concentration of HCl = 0.0800 M
Therefore, the concentration of the HCl solution is 0.0800 M.
Gas Laws and Moles
Gas laws describe the relationship between the volume, pressure, temperature, and number of moles of a gas. The ideal gas law combines these relationships into a single equation that can be used to solve problems involving moles.
Ideal Gas Law
The ideal gas law is given by the equation PV = nRT, where:
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m³)
- n is the number of moles of the gas
- R is the ideal gas constant, which is 8.314 J/(mol·K)
- T is the temperature of the gas in kelvins (K)
The ideal gas law can be used to solve for any of the variables in the equation, provided that the other three variables are known.
Example Problem
A container contains 2.0 moles of nitrogen gas at a pressure of 1.0 atm and a temperature of 298 K. What is the volume of the container?
Using the ideal gas law, we can solve for V:
“`PV = nRTV = nRT/PV = (2.0 mol)(8.314 J/(mol·K))(298 K)/(1.0 atm)V = 49.9 L“`
Therefore, the volume of the container is 49.9 L.
Practice Problems
To enhance your understanding of moles, let’s delve into a series of practice problems that cover a range of concepts, including calculations, stoichiometry, and gas laws. These problems will provide you with an opportunity to apply your knowledge and reinforce your understanding.
Solutions or answer keys are provided for each problem to guide you through the process and ensure your accuracy. Feel free to work through these problems at your own pace, and don’t hesitate to refer to the answer keys as needed.
Calculations
- Calculate the number of moles in 25.0 g of sodium chloride (NaCl).
- Determine the mass of 0.500 moles of magnesium oxide (MgO).
Stoichiometry
- Balance the following chemical equation: Fe + O2→ Fe 2O 3
- Calculate the number of moles of oxygen gas required to react completely with 2.00 moles of propane (C 3H 8).
Gas Laws
- A gas sample occupies a volume of 2.50 L at a pressure of 1.20 atm and a temperature of 298 K. Calculate the number of moles of gas present.
- A sample of nitrogen gas (N2) has a volume of 10.0 L at a temperature of 25 °C. What is the pressure of the gas if the number of moles remains constant and the volume is reduced to 5.00 L?
Clarifying Questions
What is the concept of moles in chemistry?
A mole is a unit of measurement that represents a specific amount of a substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
How do I calculate the number of moles in a substance?
To calculate the number of moles in a substance, divide the mass of the substance by its molar mass.
What is the relationship between moles and mass?
The molar mass of a substance is the mass of one mole of that substance. The molar mass is a constant for each substance and can be found on the periodic table.
How are moles used in stoichiometric calculations?
Moles are used in stoichiometric calculations to determine the amount of reactants and products involved in a chemical reaction.
What is the purpose of a titration?
A titration is a laboratory technique used to determine the concentration of a solution. It involves adding a known volume of a solution of known concentration to a solution of unknown concentration until a reaction occurs.